Moving away from our dependence on antibiotics through alternative antimicrobial strategies.
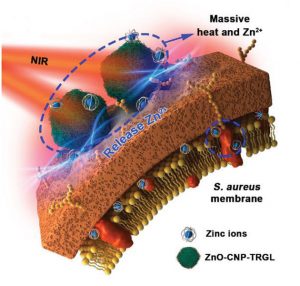
Figure 1. Proposed antibacterial mechanism of ZnO‐CNP‐TRGL nanocarbons.
Bacterial drug resistance is a global emergency. Poor practice and overuse of antibiotics have resulted in the spread of new bacterial resistance mechanisms, threatening our ability to treat common infections and diseases and leading us ever closer to a post-antibiotic era.
It goes without saying that the significance of this is quite terrifying, as this means living in a world in which even minor injuries and ailments can once again kill. Routine surgery would become too risky, organ transplants unthinkable, more women dying in childbirth, chemotherapy would become impossible—the list goes on and on.
In a recent study, authors from Sichuan University and Freie Universität Berlin propose that in order to properly address this growing threat, we need to move away from our dependence on antibiotics and toward alternative antibacterial materials and strategies.
In this vein, a flurry of research has resulted in novel chemo-, photothermal-, and photodynamic-based sterilization techniques, which provide multi-level approaches to disinfection.
Inspired by recent advances, the research team proposed constructing a near infrared (NIR)-responsive nanocarbon-based metal organic framework (MOF) with chemo-photothermal therapeutic capabilities (Fig. 1).
“MOFs are a new class of multifunctional nanomaterials, and are precursors for the synthesis of nanocarbons. They have the advantage of high photothermal conversion efficiency, easy doping of metal atoms, and large specific surface area of porous structures”, says Prof. Chong Cheng, one of the corresponding authors. “This provides a convenient strategy for [their] application in chemo-photothermal therapy to replace traditional antibiotic treatment.”
ZnO-doped carbon nanoparticles (ZnO-CNP) were first synthesized then coated with a thermoresponsive polymer gel layer, resulting in what the authors call “ZnO‐CNP‐TRGL nanocarbons”. The photothermal abilities, controlled Zn2+ ion release, and biocompatibility of these new nanocarbons were then explored by the team.
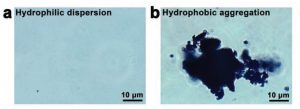
Figure 2. a) Before and b) after NIR irradiation.
Once irradiated by NIR light, heat was generated by the thermoresponsive layer and the temperature of the ZnO‐CNP‐TRGL nanocarbons increased. Under such conditions, the originally hydrophilic thermoresponsive polymer becomes hydrophobic, converting the soluble nanodispersions to micrometer aggregations, which can physically trap bacteria (Fig. 2).
These experimental results demonstrate that these nanocarbons possess an “ON-OFF” switch for chemo-photothermal disinfection. This unique transformation, from nano-dispersions to micrometer-aggregations, enables localized treatment.
The ZnO‐CNP‐TRGL nanocarbons aggregates adhere to the bacterial membrane, heating it while also releasing Zn2+ ions, denaturing membrane proteins and intracellular enzymes (Fig. 1). Before NIR irradiation, the structures of bacterial membranes are intact and no obvious Zn signal is detected from the inside area of the bacterial section, indicating a controlled release.
Perhaps most encouraging, these novel nanocarbons displayed nearly 100% bactericidal activity at very low dosage in animal models, with activity comparable to vancomycin. Upon NIR irradiation (1 W cm−2), the ZnO‐CNP‐TRGL treated abscess showed robust photothermal efficiency, with the temperature of the abscess increasing to 55 °C and no NIR response in the unaffected tissue.
These novel ZnO‐CNP‐TRGL nanocarbons have shown robust and localized chemo‐photothermal bactericidal capabilities, demonstrating safe and efficient wound disinfection. While still in its preliminary stages, this technology—and others like it—have the potential to be antibiotic alternatives.
“Our research provides numerous possibilities for the [application of] nanomaterials in broad-spectrum eradication of pathogenic bacteria with many applications such as skin pathogen infection, implant sterilization, and wastewater treatment,” says Prof. Cheng.