Using plant proteins derived from crop waste and spent grains adds new dimension to sustainable lab-grown meats.
A lot of research is going into developing lab-grown meat, with many hoping that a synthetic alternative to farmed animals might help address longstanding issues such as animal welfare, transmission of zoonotic diseases, overuse of antibiotics, and a reduction in carbon emissions.
While we are closer than ever to bringing artificial meats to the dinner table, there are still hurdles to overcome. Dejian Huang at the Department of Food Science & Technology at the National University of Singapore has been working in this field for the past decade, and is hoping to clear some of the hurdles through new and innovative approaches to creating lab-grown meat.
“Cell-based meat holds the promise of revolutionizing the meat industry for sustainable development […] and the slowing of global warming,” explained Huang. “Cultured meat can be customized for better nutritional values and variable flavors to meet the different preferences of consumers. [It] can be conducted in factories in places, such as Singapore, where the agricultural land is scarce. Lab-grown meat could be a solution to the growing food security problem.”
Getting creative with 3D printing
Cultivating meat in a lab involves far more than just stem cells. In order to gain consumer acceptance, scientists must successfully recreate not only the taste but also the shape and texture of conventional meat. Picture the unappetizing sight of a blob of cells — how likely are you to want to eat it, regardless of how good it tastes?
“There are many challenges for culturing meat from cells, and one of them is the lack of scaffolds that provide the structural support for cells to multiply and develop into tissue,” Huang added.
To build engineered tissue, biological scaffolds are required to help guide and support the cells as they grow into their 3D structures.
3D printing offers an inexpensive and time-efficient means of producing them, but not just any 3D printer would do. A specialized 3D printing technique called electrohydrodynamic printing can be used to create ultra-fine and fibrous scaffolds that are the perfect environments for growing cells to get the nutrients and support they need.
The fibers produced via electrohydrodynamic printing are used extensively in regenerative medicine as these fibrous scaffolds resemble the structure of natural muscle tissue, where cells are aligned and organized into cable-like bundles called fascicles. However, the translation to cultured meats is not completely straightforward as the scaffolds used in biomedicine are typically made from synthetic materials, such as plastics.
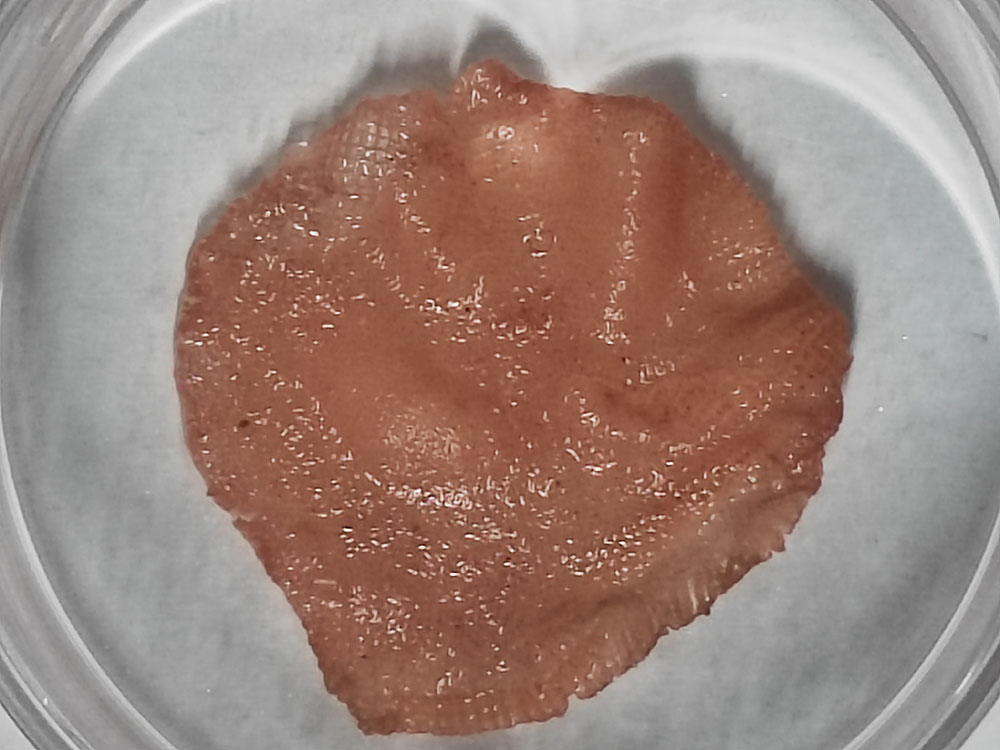
But Huang and his team were not daunted. “Using 3D-printed edible scaffolds for meat culture came naturally to us,” said Huang. “However, edible […] scaffolds remain scarce in cultured meat production.”
Animal-derived proteins are a natural candidate to make the inks necessary to build the scaffolds. But these can be expensive and Huang wanted to also address the element of sustainability in his approach.
“3D-printed plant protein scaffolds could bring new [opportunities] to develop cell-based meat with real meat appearance,” he explained. “But not only that, they provide a cost-effective, edible material to replace expensive animal-derived proteins because these plant proteins can be extracted from spent grains, such as spent corn meal in bioethanol plants and spent malt in the beer brewing industry.”
The inks and subsequently the scaffolds the team made were therefore derived from cereal proteins, like maize, barley, and rye, which are all abundant crops, added Huang.
“Hordein or secalin, from barley or rye, were mixed with zein (obtained from spent corn meal) to formulate ink with favorable printability,” he continued. “The resulting scaffolds made from the ink contains only proteins from these grains and are thus edible (although it may not be tasty). They possess high porosity and proper microstructure for cells to develop meat texture.”
Getting the public on board
More work is needed before an affordable and realistic synthetic meat can be made commercially available. In addition to hurdles related to the scalability and efficiency of 3D printing prior to commercialization, Huang emphasizes that the public needs to also get on board.
“Taste, texture, cost, and food safety are all concerns that need to be addressed,” he said. “We need to give consumers peace of mind as well as great joy of consuming lab-grown meat that are safe, tasty, and having superior nutritional value compared to conventional meat.”
Advances in 3D printing are still required to achieve mass printing of scaffolds in a cost-effective manner, as well as developing edible inks that endow the scaffolds with desired functionality. But Huang is optimistic these problems may be solved in just a few years.
He says that he and his team are not restraining themselves to just 3D printing and that they’re currently developing alternative methods to produce their structured scaffolds while bringing down the cost of manufacturing them on a commercial scale.
“To expand the scope of our technology, our lab has been working on culturing different types of animal cells, including fat cells, on the scaffolds that can be made in different shapes and plant protein composites,” he continued. “Fundamentally, we want to address the relationship between the scaffolds and culturing of various animal cells so that we can rationally design scaffolds that are optimal for culturing specific type of meats.”
Reference: Lingshan Su, Linzhi Jing, Dejian Huang, et al., 3D-Printed Prolamin Scaffolds for Cell-Based Meat Culture, Advanced Materials (2023). DOI: 10.1002/adma.202207397
Featured image: Cultured pork meat on printed scaffolds demonstration

