Using a derivative of cellulose, researchers explore making a natural food colorant from materials whose surfaces manipulate light.
Food colorants are used extensively in the food industry for various purposes, from enhancing a food’s natural pigmentation to differentiating between flavors or providing eye-catching colors to draw consumers in — anyone remember Heinz’s purple ketchup?
Just like flavor, dyes and pigments are derived from both natural and artificial sources, and while they have no nutritional benefit, they do enhance our enjoyment of food. But in recent years, consumers have tended to shy away from synthetic or artificial ingredients as a result of potential health concerns.
While limited research suggests that food dyes could negatively impact sensitive individuals, such as a child’s behavior or those prone to migraines, the overall consensus to date is that these substances are safe to consume and do not pose a health risk to the general population.
“[Nevertheless], a growing number of companies are looking to transition to exclusively natural colorants,” explained Silvia Vignolini, professor of chemistry and biomaterials at the University of Cambridge. “But this can be challenging, as natural colorants can often be more expensive and there often aren’t many suitable alternatives, especially for blue hues.”
Edible pigments from photonic materials
Vignolini and her team have been exploring the use of photonic materials as food pigments. The word “photonic” may sound artificial, but it simply refers to any material that can emit, detect, manipulate, or control light. Many examples exist in the natural world, from the vibrant wings of butterflies and the metallic scales of beetles to the pixelated appearance of certain fruits.
Taking inspiration from nature, the team explored how edible hydroxypropyl cellulose can be used to produce such pigments. Their findings were recently published in Advanced Sustainable Systems.
“Hydroxypropyl cellulose (HPC) is a derivative of natural cellulose — a highly abundant biopolymer found in plants — and is biocompatible, biodegradable, and even edible,” said Richard Parker, senior researcher at the University of Cambridge and one of the study’s authors. “It is typically used as a binder or stabilizer for foods and pharmaceuticals.”
But HPC has a unique feature that intrigued the team, as when it is dissolved in high concentrations in water it forms a liquid crystal phase, which is a state of matter between a liquid and solid crystal. This essentially means that although the material still flows like a liquid and does not form a traditional crystal lattice, its molecules may still orient themselves in a crystal-like way.
Liquid crystalline HPC has interesting optical properties as a result, displaying vibrant, iridescent colors that are dependent on its concentration in water. “This effect arises from light interference with the periodic nanostructure of liquid crystalline HPC, which leads to the strong reflection of a specific hue of light,” explained Clement Chan who was a postdoctoral researcher at the University of Cambridge when he participated in the study.
The problem is that once the HPC solution is dried, it loses its vibrant color. “There have been many attempts to maintain this color through either encapsulating the HPC solution so evaporation cannot occur, or chemically cross-linking the HPC so the structure is trapped [though this leads to distorted colors],” added Chan.
But these approaches haven’t translated into anything substantial as the scientists say it is questionable whether the intended natural properties of HPC are retained after introducing a synthetic cross-linking group, limiting its suitability as an edible photonic material.
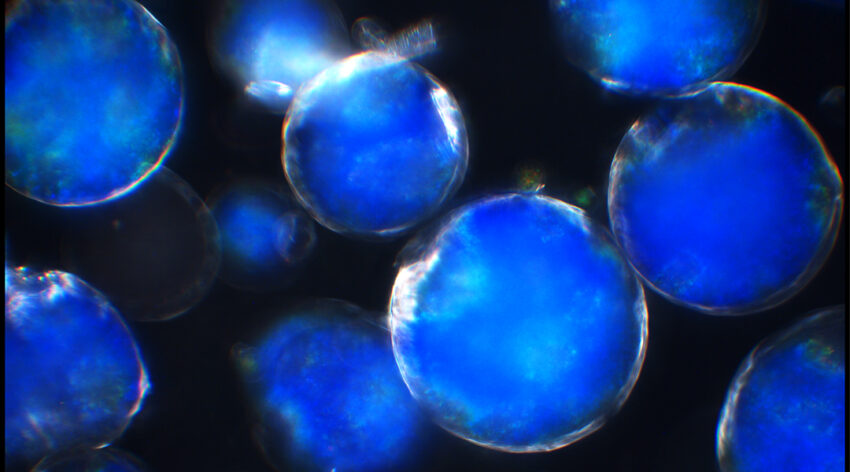
Isolating color droplets
To get around these problems, the team came up with an alternative means of producing solid HPC pigments that retain their colors. Firstly, they mixed a low concentration solution of HPC in water with oil to form an emulsion in which tiny droplets of aqueous HPC form. The water from these droplets was then removed by heating the emulsion to 68.5–77.5 °C, producing visibly red, green, and blue microparticles of pure HPC.
The final color of the HPC liquid crystal phase is dependent on both its concentration in water and temperature. “The color will shift towards red hues upon heating,” explained Vignolini. “This is important as we use this to counterbalance the strong shift towards blue (and ultimately ultraviolet) wavelengths that occurs upon drying HPC.”
“By balancing these two behaviors, we can maintain the color of HPC into the solid state without using any additional synthetic additives,” she continued. “In this way, we can tune the visual appearance of the microspheres by just ‘locking’ the color in at different temperatures, resulting in a variety of hues from a single HPC formulation.”
“By producing pure HPC pigments with a range of colors using only food-grade ingredients, we provide a scalable and cost-effective alternative that can replace these conventional colorants,” added Parker.
Reference: Silvia Vignolini, et al., Exploiting the Thermotropic Behavior of Hydroxypropyl Cellulose to Produce Edible Photonic Pigments, Advanced Sustainable Systems (2023). DOI: 10.1002/adsu.202200469