It is said that few people have saved more lives than Louis Pasteur. Though he began as a chemist, Pasteur’s career cut across disciplines, earning him recognition in fields such as microbiology, immunology, medicine, food processing, and manufacturing.
He is renowned for his work in microbial fermentation, pasteurization, and is widely credited for the germ theory of disease. He was also the first to manufacture vaccines based on attenuated, or weakened, viruses, developing the rabies and anthrax vaccines, and making major inroads into combating cholera.
This edition of Pioneers in science celebrates the life and work of this pivotal figure on the anniversary of what would have been his 200th birthday.
A formative upbringing
Born on December 27, 1822 in Dole, in the east of France, historians attribute Pasteur’s rural upbringing as a great influence on his later endeavors. This was a region in which the old, practical arts, such as wine making and tanning, were very much prominent, involving complex chemical practices that interested and stuck with Pasteur.
It’s said that as a boy, he didn’t like school very much and was a mediocre student, instead spending a lot of his time drawing, fishing, and painting. Nevertheless, after a brief initial stint at the Institution Barbet in Paris, he returned to the east of France as a result of homesickness, earning his Bachelor of Letters in 1840 and his Bachelor of Science in 1842 from the Royal College of Besançon.
In 1847, he obtained his doctorate from the École Normale Supérieure and in 1848, after working briefly as a physics professor at the Dijon Lycée secondary school, Pasteur was appointed professor of chemistry at the University of Strasbourg.
Molecules with “handedness”
Pasteur is well known within the chemistry community for his discovery of molecular asymmetry, namely, that a molecule made up of the same connected atoms can exist in different spatial orientations, and in some cases, can be mirror images of one another — a property known as chirality.
This came about as a result of his experiments with a group of molecules called tartrates, which are found in the sediments of fermented wine.
In chemistry, a means of studying molecules is through analyzing their effect on the rotation of polarized light (light that moves in only one direction). When polarized light was passed through a solution of tartaric acid, the angle of the light was rotated. However, paratartaric acid, an isomer of tartaric acid, meaning it has the same chemical formula but different arrangements of atoms, did not result in any rotation. Though most scientists at the time assumed the two chemicals were identical, Pasteur deduced that they somehow had different 3D structures as a result of this difference in optical activity.
He reasoned that these molecules had a unique and “asymmetric” internal structure that was responsible for the twisting of light. Under the microscope, he observed two different types of crystals of paratartaric acid and that they appeared to be mirror images of one another.
He set about sorting the crystals into two piles and made solutions of each. When polarized light was passed through the individual solutions, they each rotated the light in opposite directions, and when a solution in which both sets of crystals were combined was used, the effect was canceled as a result of the 50/50 mixture where the crystals cancelled out each other’s light rotating abilities.
This first experiment became the foundation of a new area in organic chemistry called stereochemistry. This was a revolutionary idea at the time, as it also corroborated a theory that Pasteur had been developing: that symmetry is the key to life and that all life processes stem from the precise and asymmetrical arrangement of atoms within biological molecules.
“Only products originating under the influence of life are asymmetrical because they developed under the influence of cosmic forces which were themselves asymmetrical,” said Pasteur, where asymmetry was thought to be the major dividing line between the organic and mineral worlds — and thus the secret to life.
Fermentation and germ theory
In 1856, when Pasteur was working as dean of the Faculty of Sciences at the University of Lille, he was asked for help by local wine manufacturers to overcome the problem of alcohol souring.
A prevailing view at the time was that fermentation — the chemical breakdown of a substance — was a decomposition process perhaps initiated by yeasts, which were understood to be “alive”, but occurred as a result of instabilities arising in molecules in fermentation liquors.
But amyl alcohol recovered from fermented alcohols did not fit with this rule. Given the molecules’ asymmetry deduced through its ability to rotate polarized light, Pasteur concluded that life had somehow intervened during the fermentation process and contributed to their formation.
This view, however, was not accepted by his peers, who insisted the process was not a biological one. Thus Pasteur set about proving that fermentation occurred as a result of living microorganisms.
In his 1857 report on lactic fermentation, Pasteur reported the accumulation of material: “Under the microscope it is seen to form tiny globules or small objects which are very short, isolated or in groups of irregular masses. These globules are much smaller than those of beer yeast and move actively by Brownian movement.”
In addition, he argued that if lactic fermentation were a straightforward chemical reaction, it should lead to only the reactants and products of the said reaction. However, he found that although lactic acid was indeed the principle component, it was “far from the only product” with various alcohols and other materials always found in the fermentation mixtures.
Another prevailing theory was that of “spontaneous generation” which held that life could arise spontaneously from non-living matter, which was also a contending culprit behind wine spoiling. These were the early days of germ theory — a theory in which tiny living microbes too small to see with the naked eye exist, essentially, everywhere.
But many of Pasteur’s contemporaries disagreed with this idea, and by 1860 the debate had become so heated that the French Academie des Sciences held a contest and offered a prize for any experiments that could decisively prove or disprove spontaneous generation.
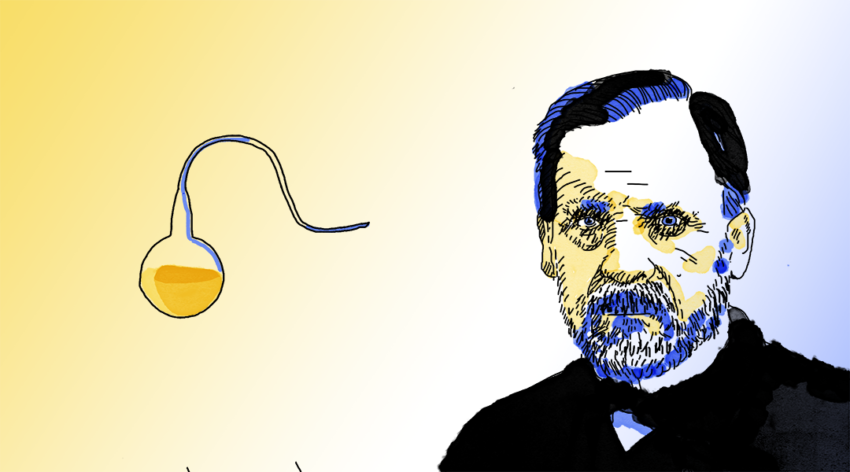
A series of experiments were compiled into an essay titled, Memoire sur les corpuscles organizes qui existent dans l’atmosphere [Memoir on the organized corpuscles that exist in the atmosphere], which he submitted in 1861. In one experiment in particular, he used his now famous swan-necked flasks to exclude air from boiled infusions, then no “living microorganisms would appear, even after months of observation. However, if atmospheric dust were then introduced, living microbes would appear within 2–3 days.”
Through this “unassailable and decisive” evidence, he was able to finally refute spontaneous generation and demonstrated the existence of tiny microorganisms. In 1864, he invented pasteurization — a heat-treatment that destroys pathogenic microbes to preserve foods such as beer, milk, and cream without harming the nutritional value or taste.
This was a major breakthrough in protecting public health as in the early 19th and 20th centuries, prior to pasteurization, tuberculosis, Q fever, diphtheria, severe streptococcal infections, and typhoid fever, among others, were common dangers associated with dairy consumption. Since then, bacterial disease outbreaks associated with milk have fallen dramatically, with many experts projecting that millions of lives have been saved as a result.
Over the next 20 years, Pasteur continued to help solve problems faced by the dairy, silk worm, wine, vinegar, and beer industries, earning widespread fame and becoming something of a French hero.
The first vaccines
Following his successes in microbial fermentation and the application of germ theory, Pasteur was inspired to apply this knowledge to the management of infectious diseases; a growing problem at the end of the 19th century due to the growth of towns and industrialization.
“The only way currently available to science to experimentally prove that a microscopic organism is the cause of both the illness itself and its transmission, is to subject the microbe to serial cultures,” he wrote in 1878.
In laboratory experiments, Pasteur attempted to alter a pathogen’s virulance or severity by passing it through different animals. His first breakthrough came in the late 1870s, when after exposing chickens to an attenuated form of the pathogen that caused chicken cholera, they become resistant to the actual virus.
In 1880, he presented his findings to members of the academy, referencing this phenomenon as “vaccination” in reference to work by Edward Jenner nearly 100 years before where “even before Jenner demonstrated the efficacy of vaccinia, people of the countryside where he practiced already knew that cowpox protected against smallpox.”
He extended his theory, stating that he believed that all microbes could be attenuated through his “atmospheric attenuation method” in which a microbe was weakened as a result of exposure to atmospheric oxygen during 2–3 month culture intervals though he never explained why oxygen weakened microbes. These weakened live microbes were then used to help the host build resistance — a concept now well known as immunity.
As such, he turned his attention to anthrax, a devastating disease in cattle and other livestock caused by the bacterium Bacillus anthracis. The German physician Robert Koch had already been working in this area and had demonstrated that the bacteria, observed under the microscope as inert rods, had a life cycle and underwent division. Building on this work and over the course of several years, Pasteur developed attenuated forms of the pathogen for use in vaccines and dramatically reduced cattle mortality.
There is some controversy around the claim that Pasteur developed the first anthrax vaccine. Pasteur was apparently very secretive about its preparation and use in his famous 1881 animal trial, eluding that he had used the methods that he had already reported for his chicken cholera vaccine in which he had used a live, attenuated virus.
It did not emerge until 1995, after examination of Pasteur’s notebooks by Gerald Geison, a professor of history at Princeton University and biographer of Pasteur, that he did not in fact use the live vaccine, but instead, had used a vaccine developed by his rival, veterinarian Jean Joseph Henri Toussaint, who had created his vaccine by simply killing the bacteria with heat before administration.
Tousssaint, unfortunately, gained no recognition for his work and died in 1890 from a mental breakdown. But in 1998, the French government officially and rightly recognized Toussaint’s vaccine as the first effective vaccine against anthrax.
The rabies vaccine
Shortly after his very public success with the anthrax vaccine, he spoke to the International Congress of Directors of Agronomic Stations in Versailles and expressed the intent to apply his attenuation method to an unknown microbe found in the saliva of a child who had died of rabies.
Rabies is a viral infection and at the turn of the 19th century, had a mortality rate of almost 100%. The challenge with a virus versus a bacterium is the inability to culture and isolate it in the same way. The concept of viruses was still unknown at the time, and it wouldn’t be until the 1930s that the first virus would be observed. This also meant that his atmospheric attenuation method could not be used to weaken the virus for inoculation.
Over the next few years, Pasteur experimented with methods of serial passage of the rabies virus through different species to determine whether he could attenuate its virulence. In 1884, he reported that the passage of the virus from dogs through monkeys attenuated it when it was re-administered to dogs, eventually showing that he could immunize dogs against rabies.
In July of 1885, Pasteur took on the case of Joseph Mesiter, a nine-year-old boy who had been bitten by a rabid dog. His mother had heard of Pasteur’s work in developing a cure for rabies and took him to Paris where, after a series of injections consisting of dissected nervous tissue over several weeks, Mesiter survived.
This sent a shock wave through not just the medical world, but the public in general, with the achievement making it to the front page of numerous French newspapers. In 1885, Pasteur’s rabies vaccines became widely used, with people reportedly traveling from as far as India to receive the treatment.
After this presentation to the academy, Pasteur gradually withdrew from active experimentation, until his death in 1895 at age 73. His contributions to society were far reaching, demonstrating the application of science at its best.
“Science knows no country, because knowledge belongs to humanity, and is the torch which illuminates the world” – Louis Pasteur.
Read more Pioneers in science stories here
Illustration by Kieran O’Brien