A new hydrogel supports living cells and can withstand dynamic environments to help repair tissue in the heart, muscles, and vocal cords.
A rendered image of the vocal cord bioreactor for testing the hydrogels. Image credit: Zixin He
Medicine has been evolving for thousands of years, but it’s only been recently that we have been able to harness microscopic biological mechanisms to not only improve therapeutic intervention, but to understand underlying causes of disease.
Instead of just treating disease or injury, regenerating damaged organs and tissues has been a long-held dream. This area of research began to take shape in the early 2000s with the advent of stem cell therapies and advancements in tissue engineering. Central to this area of research have been materials called hydrogels, which are water-absorbing polymer networks that mimic the physical, chemical, and biological environments of living tissues.
They have been pivotal in carrying this field forward, serving as drug delivery platforms, tissue mimics, and disease models. But there have been roadblocks hindering them reaching their full potential.
“There are two challenges in the field,” says Guangyu Bao, a Ph.D. candidate in the Department of Mechanical Engineering at McGill University. “First, most injectable hydrogels are not porous, and because of this, nutrients and oxygen can only diffuse less than 1 millimeter into most hydrogels. This range is not enough when tissues and organs are larger than several centimeters.
“Second, most injectable hydrogels cannot survive highly dynamic environments. An extreme case is the human vocal cord, where the organ vibrates hundreds of times per second. This poses challenges to the mechanical stability of injected hydrogels.”
Porous and tough hydrogels are the best of both worlds
Bao is corresponding author on a paper published in Advanced Science — along with Luc Mongeau and Jianyu Li — in which a new porous hydrogel is reported that is tough enough to repair challenging, moving organs, such as the heart, muscles, and vocal cords.
Porous hydrogels have been developed before and serve an important purpose: they are more readily injected — i.e., not damaged when they pass through a needle — and better promote the diffusion of nutrients to help implanted cells survive. But there is understandably a tradeoff as the more porous a hydrogel, the more readily it will fall apart under mechanical stress.
Bao and colleagues turned to double network hydrogels, a special hydrogel that consists of two types of polymers: a rigid, crosslinked polymer and a flexible, ductile polymer. This affords them a high degree of toughness while still maintaining a soft consistency, comparable to rubber. Current examples therefore solve the mechanical stability problem, but their low porosity makes them unsuitable for injection or supporting living cells.
The team therefore developed their own version, which they called a “porous double network” hydrogel. It contains both a rigid and flexible component, with the caveat that it forms interconnected, cell-sized pores upon injection. “Unlike many porous hydrogels that are weakened by their pores, [our double network hydrogels] are tough and resilient […] despite the presence of defect-like pores,” they wrote in their paper.
A one-stop treatment for tissue repair
The hydrogel takes advantage of the phase separation of a polysaccharide called chitosan, found in the skeleton of shellfish. At body temperature and physiological pH, chitosan polymers separate into a polymer-rich and polymer-poor layer, the latter of which is mainly water — and perfect for growing cells. This results in the formation of large pores after injection that were found to promote cell growth and exhibited diffusion beyond 60 mm in organ models — the largest value reported in the literature.
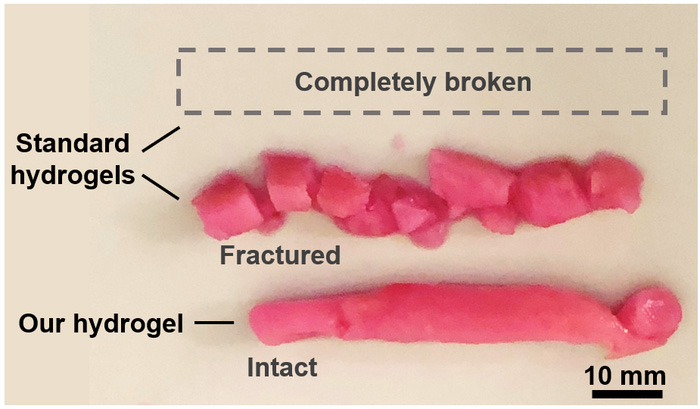
“Our goal is to offer a one-time treatment to permanently repair damaged tissues,” said Bao. To test this, the team applied their hydrogel to a vocal cord model and found it to be stable even after six million mechanical stimulations.
Bao says they will next test biocompatibility, degradation, and therapeutic effects in soft tissue repairs: “We are ready to take on new challenges and look forward to the next chapters of our technology’s development.”
“Our work highlights the synergy of materials science, mechanical engineering and bioengineering in creating novel biomaterials with unprecedented performance,” said Li in a statement. “We are looking forward to translating them into the clinic.”
Reference: Sareh Taheri, et al., Injectable, Pore-Forming, Perfusable Double-Network Hydrogels Resilient to Extreme Biomechanical Stimulations, Advanced Science (2021). DOI: 10.1002/advs.202102627

